The evolution of peptide microarray technology: From traditional methods to modern laser printing
Article

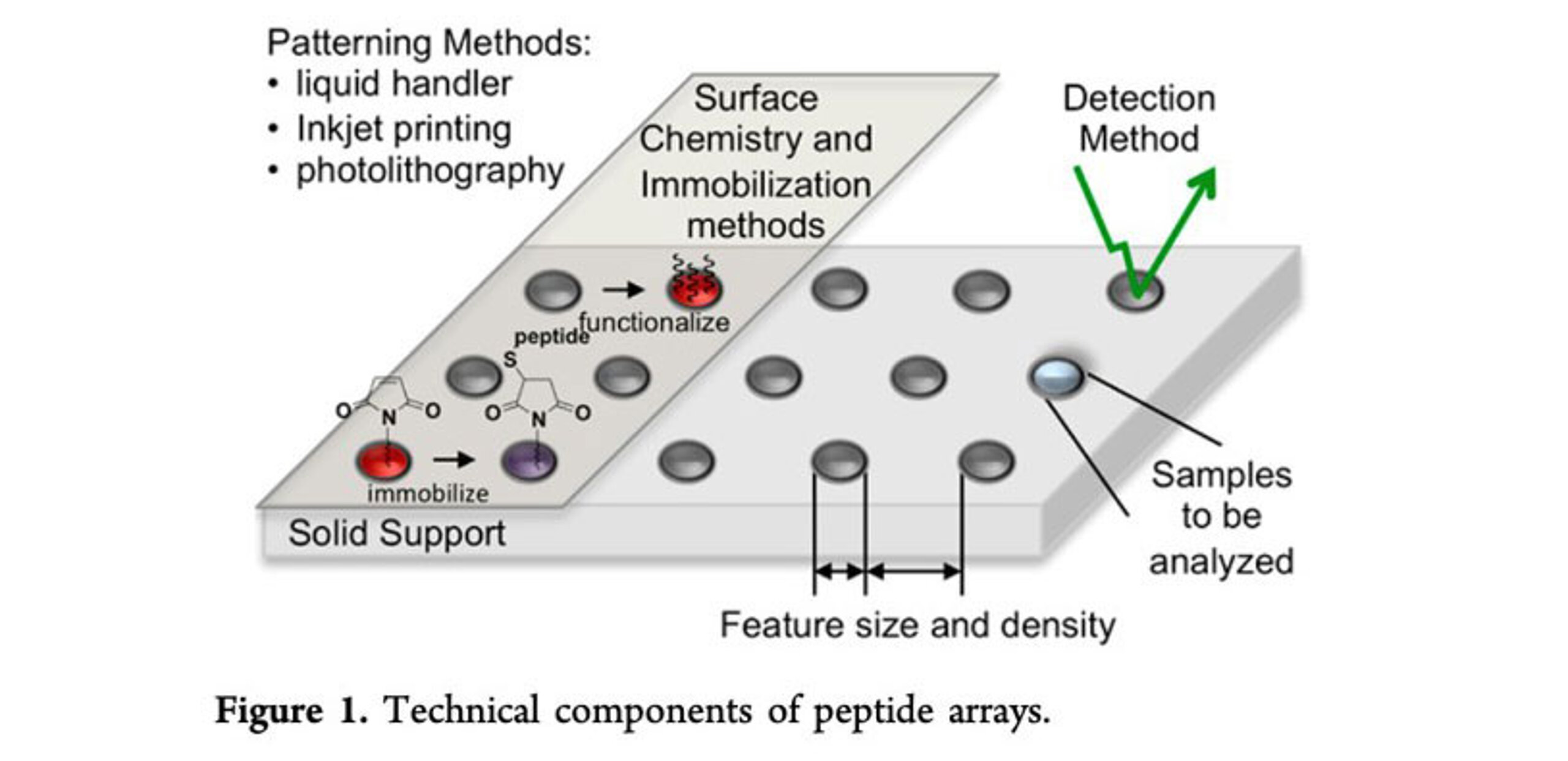
Peptide microarrays are powerful tools that allow the simultaneous analysis of thousands of peptides in a single experiment. They have become invaluable in biotechnology and pharmaceutical research, enabling applications from epitope mapping and immune profiling to antibody validation and drug discovery.
Over the past few decades, peptide microarray technology has evolved dramatically. Early approaches in the 1980s and 1990s relied on laborious parallel synthesis or manual spotting of pre-synthesized peptides, yielding low-density arrays. Today, state-of-the-art platforms like PEPperPRINT’s laser-based printing technology can produce high-density peptide microarrays rapidly and cost-effectively, with a flexibility and scale that were previously unachievable.
This article provides a historical overview of that evolution, from the traditional methods to the modern laser printing techniques.
Early pioneers in parallel peptide synthesis (1980s)
Long before “peptide microarrays” existed in their current form, researchers sought ways to synthesize and test many peptides in parallel. In the mid-1980s, Mario Geysen introduced a groundbreaking “multipin” ELISA method or epitope mapping. In this approach, dozens of peptides could be synthesized simultaneously on the tips of plastic pins (each pin acting as an individual solid support), and then screened for binding to antibodies. Around the same time, Richard Houghten developed a tea-bag-based combinatorial synthesis method to generate multiple peptides in parallel.
These pioneering techniques demonstrated the proof-of-concept of high-throughput peptide synthesis and screening, allowing identification of viral antigen epitopes using many peptides tested in one experiment. However, they were not “microarrays” on a planar surface, and each peptide was handled separately, limiting scalability.
Solid-phase peptide synthesis (SPPS), first introduced by R. Bruce Merrifield in 1963, was the foundation of all these methods. Merrifield’s method attaches a growing peptide to an insoluble resin, enabling iterative coupling of amino acids followed by routine washing and deprotection steps. Early parallel approaches like Geysen’s and Houghten’s essentially multiplied the number of resin supports or reaction vessels so that many peptides could be built at once. While SPPS yields high-quality peptides, synthesizing hundreds or thousands of distinct sequences individually was still time-consuming and expensive.
These early innovations showed it was possible to parallelize SPPS, but the need remained for a more efficient, miniaturized format where peptides could be synthesized and tested on a single planar substrate.
The SPOT method on membranes (1990s)
A major leap toward true peptide arrays came with the development of the SPOT synthesis technique by Dr. Ronald Frank in 1992. Frank’s SPOT method enabled the in situ synthesis of peptides on a flat surface (originally cellulose membrane) in a grid-like pattern of spots. Using this approach, droplets containing activated Fmoc-protected amino acids are manually or robotically spotted onto defined locations on a derivatized membrane. Each spot on the membrane functions as a microscopic reaction chamber where a peptide is elongated stepwise: the cycle of spotting an activated amino acid, coupling, washing, and deprotecting is repeated for each amino acid in the sequence. After all coupling cycles, the membrane contains an array of peptides attached at the marked spots.
The SPOT method was revolutionary because it eliminated the need for separate reaction vessels for each peptide: everything happened on one surface, dramatically reducing reagent usage and cost per peptide. It became a gold standard for peptide arrays throughout the 1990s and 2000s, due to its relative simplicity and reliability. Peptides up to 20–25 amino acids long could be synthesized on the membrane. Frank and colleagues demonstrated that SPOT-synthesized peptide membranes were powerful for applications like scanning protein sequences to map antibody epitopes or enzyme substrates. Membrane-bound peptide arrays were later commercialized by some companies, making epitope mapping kits available to many labs.
Despite its success, the original SPOT synthesis had some limitations. One major drawback was the relatively low density of peptides that could be achieved on a membrane surface. The spots of amino acid solution tend to spread, limiting the packing density to roughly ~25 spots per cm² in the traditional setup. This translates to only a few hundred peptides on a reasonably sized membrane, which in turn limits the throughput and increases the cost per experiment if very large libraries are needed. Additionally, cellulose membranes themselves could be problematic for certain assays as they are opaque (not suitable for fluorescent readouts) and can nonspecifically bind assay proteins, causing higher background. Last but not least, once dissolved, any excess of activated amino acid could not be reused, disadvantageous for material needs and costs.
Throughout the 1990s, SPOT and its variants remained widely used. Automated instruments were introduced to perform SPOT synthesis, and improvements like cellulose derivatization and better coupling chemistry enhanced peptide yields. By the early 2000s, SPOT synthesis had firmly established the principle that hundreds of peptides could be synthesized in parallel on a planar surface. The stage was set for further miniaturization and for moving peptide arrays onto higher-density glass slide formats, compatible with the emerging microarray analysis technologies (e.g. fluorescence scanners) that had been developed for DNA microarrays.
Transition to glass slides: Early peptide microarrays (2000s)
In the late 1990s and early 2000s, as DNA microarrays became popular, researchers began adapting microarray printing techniques to create peptide microarrays on glass slides. This approach did not synthesize peptides in situ on the surface, but rather relied on pre-synthesized peptides that were printed and immobilized in array format (an ex situ method). Peptides could be synthesized by conventional SPPS (or via SPOT on a large scale), then purified and spotted onto a chemically coated slide using a robotic microarrayer with tiny pins. Each pin would deposit a small droplet of peptide solution at a defined coordinate on the slide, where the peptide would attach (e.g. via covalent linkage to reactive groups on the slide, or by adsorption onto a nitrocellulose-coated slide). By printing many peptides in an array, researchers could create custom peptide microarrays for screening.
This pin-spotting method was leveraged by established DNA/protein microarray equipment, allowing laboratories or companies to produce peptide microarrays without developing a completely new synthesis technology. Companies started offering arrays of hundreds to a few thousand peptides created by immobilizing pre-made peptides on glass slides. Such arrays proved useful for biomarker discovery, immune monitoring, and interaction studies. Another advantage is that any peptide or peptide analog could be printed, including those with post-translational modifications or non-natural residues. Furthermore, because the peptides are purified beforehand, the array quality was high (each spot contains predominantly the intended peptide).
However, the ex situ printing approach also had trade-offs. Preparing large numbers of different purified peptides was still expensive and time-consuming for very high-density arrays. While printing resulted in higher densities than SPOT on a membrane, it still falls short of the ultra-high densities that modern in situ synthesis can reach.
Despite these challenges, pin-printed peptide arrays became a mainstay for many mid-throughput applications in the 2000s, because they allowed researchers to leverage peptide libraries with known sequences and high purity. This period saw growing adoption of peptide arrays in research and even early commercial diagnostic efforts, though the arrays were relatively low-density by today’s standards.
Photolithography-based in situ synthesis (1990s–2000s)
While SPOT and pin-based synthesis methods dominated the early landscape of peptide microarray technologies, the 1990s saw the introduction of a radically different technique: photolithography, a technique inspired by methods used in semiconductor manufacturing. In 1991, Stephen Fodor and colleagues published a foundational paper demonstrating spatially addressable, light-directed parallel chemical synthesis. Although initially focused on oligonucleotides, this approach was soon explored for peptide arrays as well.
The method works by using light to control where amino acids are added on a slide. The surface is initially coated with special chemical groups that block synthesis. By irradiating UV light in a specific pattern, these blocking groups are removed only in the exposed areas. Then, a selected amino acid is added and binds only to these unblocked spots. By repeating this process — changing both the light pattern and the amino acid — researchers can build thousands of different peptides in parallel, each in a specific location on the surface.
The main promise of this method was ultrahigh feature density, enabling the synthesis of hundreds of thousands of peptides per array, far exceeding the throughput of contact or SPOT-based methods.
Despite its technical sophistication, photolithographic synthesis of peptide microarrays faced significant limitations. The main bottleneck is the need for a large number of synthesis cycles due to the sequential coupling of each amino acid for each position. This made the synthesis process highly laborious and time-consuming; one would need 20 x 15 = 300 coupling cycles for a peptide array of 15-mers.
Another serious technical challenge is the low deprotection efficiency of the light-sensitive protecting groups (often <80% efficiency), resulting in truncated sequences and synthesis artifacts.
Additionally, throughput remains limited. Since each array must be synthesized sequentially using specialized equipment, production is inherently slow and inflexible. The requirement for expensive and complex instrumentation adds to the barrier to adoption.
Even so, photolithographic methods contributed significantly to high-density peptide array development and remain valuable in specialized settings. However, their limitations have left room for more agile and scalable technologies to emerge in the 2010s.
Laser printing of peptides: Toward high-density and rapid production (2010s)
By the mid-2000s, it was clear that an ideal peptide microarray technology would combine the flexibility of SPOT with a higher miniaturization capacity, while reducing cost and complexity. Two key innovations emerged to meet this need: particle-based laser printing and laser-induced transfer techniques. Both approaches took inspiration from other industries (printing and laser material processing) and applied them to peptide synthesis.
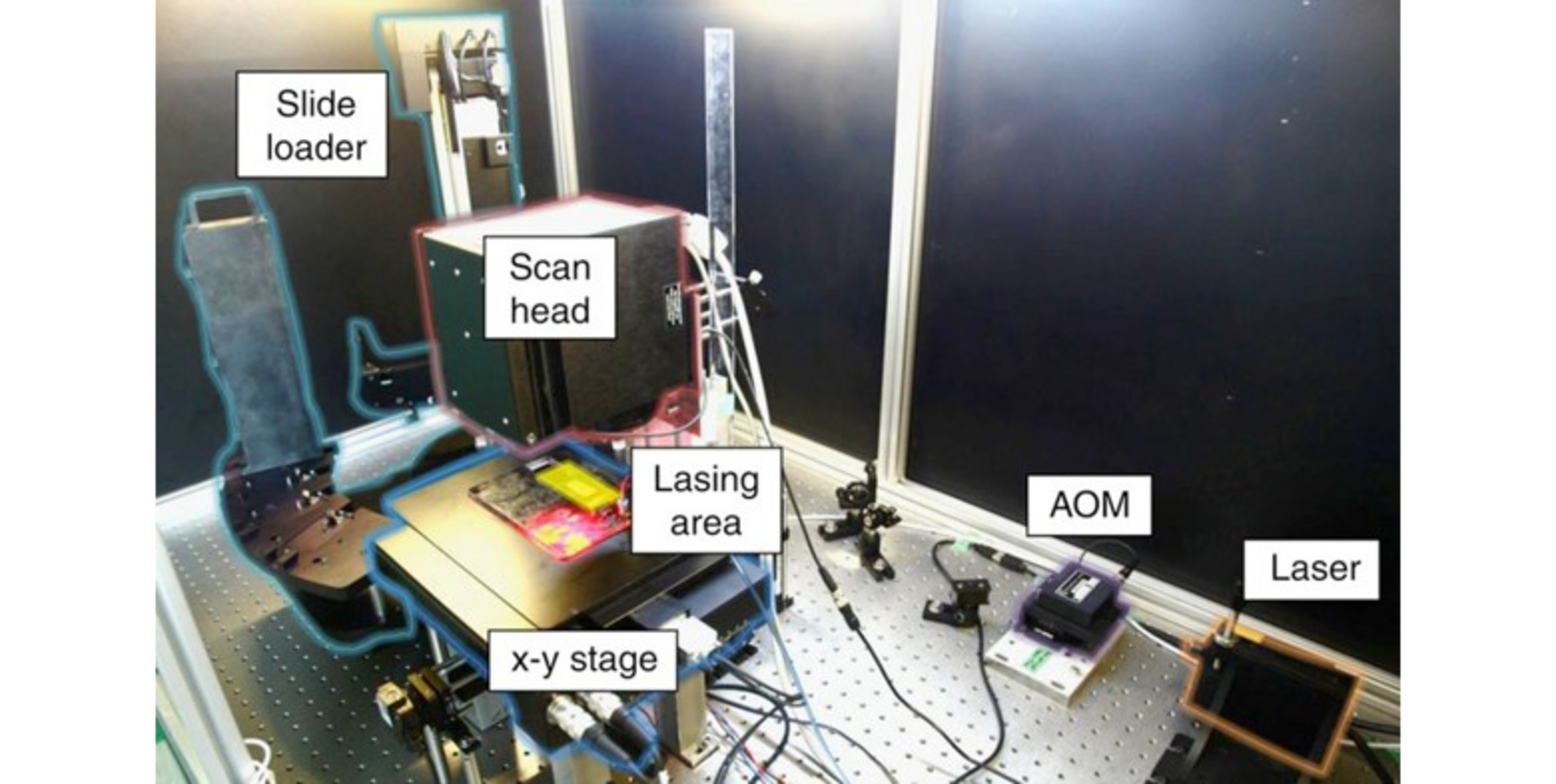
The concept of using a laser printer for peptide synthesis was pioneered by a team at the German Cancer Research Center (DKFZ) led by Dr. Frank Breitling, Dr. Ralf Bischoff, Dr. Volker Stadler, and colleagues. They developed a method in which amino acids embedded in tiny polymer particles (“toner”) could be printed onto a slide in a way analogous to how a laser printer deposits toner on paper.
The technology works roughly as follows: Each amino acid (Fmoc-protected and activated for coupling) is formulated as a dry powdered microparticle combined with a chargeable polymer matrix. A custom-modified laser printing instrument is equipped with multiple “toner cartridges,” one for each amino acid building block. Under software control, the printer propels these amino acid particles and electrostatically patterns them onto specific positions on a glass slide (which has been coated to present reactive groups for peptide coupling). After a printing cycle deposits all desired amino acids at all their respective locations on the slide, the slide is briefly heated in an oven. Heating causes the polymer particles to melt and releases the embedded amino acids, which then couple to the surface at those spots. Subsequent washing removes excess reagents, and a standard deprotection step removes the N-terminal Fmoc groups. The slide is then ready for the next cycle of printing of the next layer of amino acids.

This laser printer approach essentially adapts solid-phase peptide synthesis to a printer format. Importantly, because the amino acids are delivered as solid microdroplets, they do not spread or evaporate like liquid droplets would. This enables a dramatic increase in spot density – the printed spots can be very small and closely spaced without bleeding into each other, thus achieving spot densities orders of magnitude higher than classical SPOT membranes, while using minimal reagent volumes. In addition, the activated amino acids remain stable in the dry toners for months, significantly reducing material needs and costs.
Following their initial publications, the DKFZ team spun out the company PEPperPRINT GmbH in 2001, which became fully operative and commercialized the laser printer peptide microarray technology in 2010. By 2011, PEPperPRINT had developed a second-generation high-throughput peptide laser printer and launched routine production of high-density peptide microarrays as a product and for research services. Today, PEPperPRINT’s platform represents the state-of-the-art in peptide microarray technology. It is essentially the only broadly available technology that can rapidly synthesize large numbers of peptides de novo on glass slides in a high-throughput manner.
PEPperPRINT’s proprietary instrument is a massive, industrial-grade machine (approximately 4.5 m in length, weighing 3.5 tons) capable of printing up to 300,000 peptide spots per minute. It contains 24 amino acid toner cartridges (the 20 natural amino acids plus specialty ones). That way, custom peptide microarrays can be manufactured with unprecedented speed and density, from design to a ready-to-use microarray slide in as little as a few weeks.
cLIFT: The future of peptide microarrays (2020s and beyond)
As the demand for high-density, high-fidelity peptide microarrays continues to grow, traditional synthesis methods face limitations in scalability, resolution, and flexibility. To overcome these challenges, researchers have developed combinatorial Laser-Induced Forward Transfer (cLIFT), a cutting-edge technology that promises to revolutionize peptide microarray fabrication in the years to come.
Source: Loeffler FF, et al. High-flexibility combinatorial peptide synthesis with laser-based transfer of monomers in solid matrix material. Nat Commun. 2016 Jun 14;7:11844. doi: 10.1038/ncomms11844. PMID: 27296868; PMCID: PMC4911634.
cLIFT builds upon the principles of Laser-Induced Forward Transfer (LIFT), a technique where laser pulses transfer material from a donor to an acceptor substrate. In the context of peptide microarrays, cLIFT utilizes this approach to deposit activated amino acid monomers embedded in a solid matrix onto a glass slide. This method allows for the in situ synthesis of peptides with high spatial resolution and density, enabling the generation of microarrays with over 17,000 spots per square centimeter.
The implementation of cLIFT technology has profound implications for various fields. For instance, researchers have utilized cLIFT to synthesize arrays of neo-glycopeptides, allowing the study of multivalent glycan interactions with lectins such as concanavalin A and human langerin.
As cLIFT technology continues to evolve, its integration with other analytical techniques and automation platforms is anticipated to further enhance its capabilities. The potential to synthesize complex peptide structures with high precision positions cLIFT as a cornerstone technology in the future of proteomics and personalized medicine.
The increasing importance of peptide microarrays
One reason peptide microarrays have gained traction is their broad utility across biology and medicine. Unlike protein microarrays (which must print intact proteins that can be difficult to produce and maintain), peptide arrays present small, stable fragments of proteins and are often more robust and reproducible. In addition, post-translational modifications can be implemented in a defined and reliable manner.
Overall, peptide microarrays provide a versatile platform for any application requiring the testing of many peptide sequences in parallel, such as antibody validation or anti-drug antibody epitope mapping. They have been successfully applied in fields as diverse as immuno-oncology, infectious disease, autoimmunity, and neurology. Given their ability to compress thousands of experiments into one, they offer both discovery power and efficiency.
As we move forward, peptide microarrays will play an even greater role in life science research. Whether it’s rapidly responding to a new pandemic threat by mapping antibody targets, discovering the epitopes of autoantibodies, developing new biosimilars, or identifying new peptide drugs, this technology provides a window into the molecular interactions at the core of diseases. With continuous improvements in synthesis techniques and growing adoption, peptide microarrays have firmly established themselves from their humble beginnings to an indispensable tool of modern biotechnology.

