Decoding immunogenicity: The role of anti-drug antibodies in therapeutic design
Article
It’s no secret that modern biopharmaceuticals such as therapeutic proteins, monoclonal antibodies, and gene-editing technologies have revolutionized medicine. However, these breakthroughs come with a critical challenge: the risk of immunogenicity. When a patient’s immune system identifies a biological drug as a foreign entity, it can trigger an immune response leading to the production of anti-drug antibodies (ADA). ADA can neutralize the drug’s effect, reduce its efficacy, and, in some cases, lead to severe adverse reactions.
For pharmaceutical developers, identifying and understanding ADA responses early in the drug development process is critical. One of the most effective ways to achieve this is through ADA epitope mapping, which allows researchers to identify immunogenic regions within a therapeutic protein. These regions can then be used to improve drug design or stratify patients in clinical trials. This article explores the role of ADA, their implications for drug development, and how ADA epitope mapping can help mitigate immunogenicity risks.
Understanding ADA, T cells, and their impact on drug development
ADA can be broadly categorized into two types:
- Binding ADA: These antibodies recognize and bind to a therapeutic drug but do not necessarily neutralize its function.
- Neutralizing ADA: These antibodies block the biological activity of the drug, often leading to reduced efficacy or complete loss of function.
The presence of ADA can lead to several challenges, including:
- Reduced drug efficacy: ADA can clear therapeutic proteins from circulation more rapidly, lowering their therapeutic benefit.
- Adverse effects: Some ADA can lead to immune-mediated reactions, including hypersensitivity and organ damage.
- Increased dosing requirements: Higher drug doses may be needed to compensate for ADA neutralization, increasing treatment costs and potential toxicity.
- Long-term immunogenicity risks: Persistent ADA responses may trigger chronic immune activation, which can have significant implications for patient health.
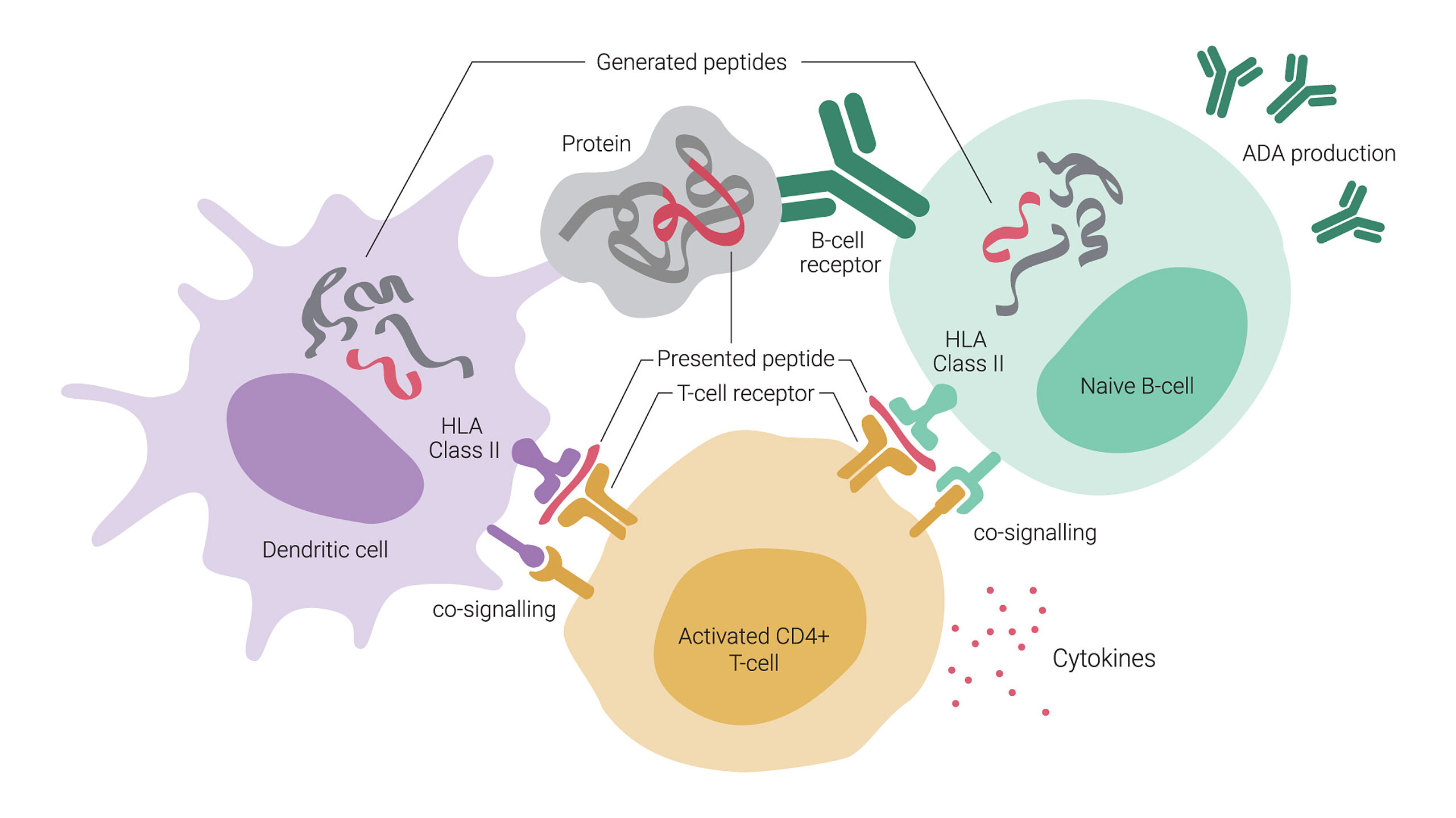
One of the critical mechanisms behind ADA generation involves T cells, particularly CD4+ T helper cells. When a biological drug is administered, antigen-presenting cells (APCs) process the therapeutic protein and present its peptide fragments on major histocompatibility complex (MHC) class II molecules. These presented epitopes activate CD4+ T helper cells, which in turn provide signals to B cells, promoting their differentiation into plasma cells that produce ADA. This interaction is crucial for generating high-affinity ADA, such as IgG, which are often associated with reduced drug efficacy and increased adverse effects. Because of this, strategies that minimize T cell recognition of therapeutic proteins are essential for reducing immunogenicity.
Why ADA considerations should begin early
Many biopharmaceutical companies assess ADA responses only in the later stages of drug development, often when clinical trials uncover unexpected immunogenicity challenges. However, ADA detection and screening should be viewed as part of a broader immunogenicity risk assessment strategy, which aims to ensure drug safety and efficacy from the earliest stages of development.
Integrating ADA risk assessment early in the preclinical phase offers several advantages:
- It allows for improvements in drug design by identifying and reducing immunogenic regions, thereby enhancing therapeutic efficacy.
- Early assessment minimizes the likelihood of clinical trial failures due to unforeseen immune responses, which can save time and resources.
- It also provides an opportunity to identify patient populations that may be less prone to developing ADA, ensuring better treatment outcomes.
Furthermore, integrating immunogenicity risk assessment allows researchers to predict potential immune responses, refine drug formulations, and implement necessary modifications in the manufacturing process to proactively mitigate risks. This approach ultimately leads to a safer and more effective final product by reducing the likelihood of ADA-related complications.
ADA epitope mapping: A key solution and the role of T cells
An effective strategy for managing ADA risks is epitope mapping. As an early-stage analysis, it can be used to detect pre-existing immune responses to the drug candidate. In later stages of drug development, it can be used to monitor treatment-emergent immune responses or to improve patient stratification in clinical trials.
ADA epitope mapping can be easily performed with peptide microarrays. The approach involves turning the therapeutic protein’s amino acid sequence into an overlapping peptide library, which is then immobilized onto a microarray. By incubating these peptides with patient or healthy control sera, we can identify specific epitopes that trigger an immune response.
Our peptide microarray was used to map the B-cell epitopes targeted by antibodies in the pooled sera from each group of vaccinated mice. The array featured cyclic overlapping peptides — short sequences of amino acids arranged in a closed loop structure that overlap with each other — covering the full spike protein of the SARS-CoV-2 Index strain and including key mutations. This setup helped identify the specific virus regions recognized by antibodies, enabling researchers to map out immune responses and identify potential areas crucial for designing vaccines effective against multiple variants.
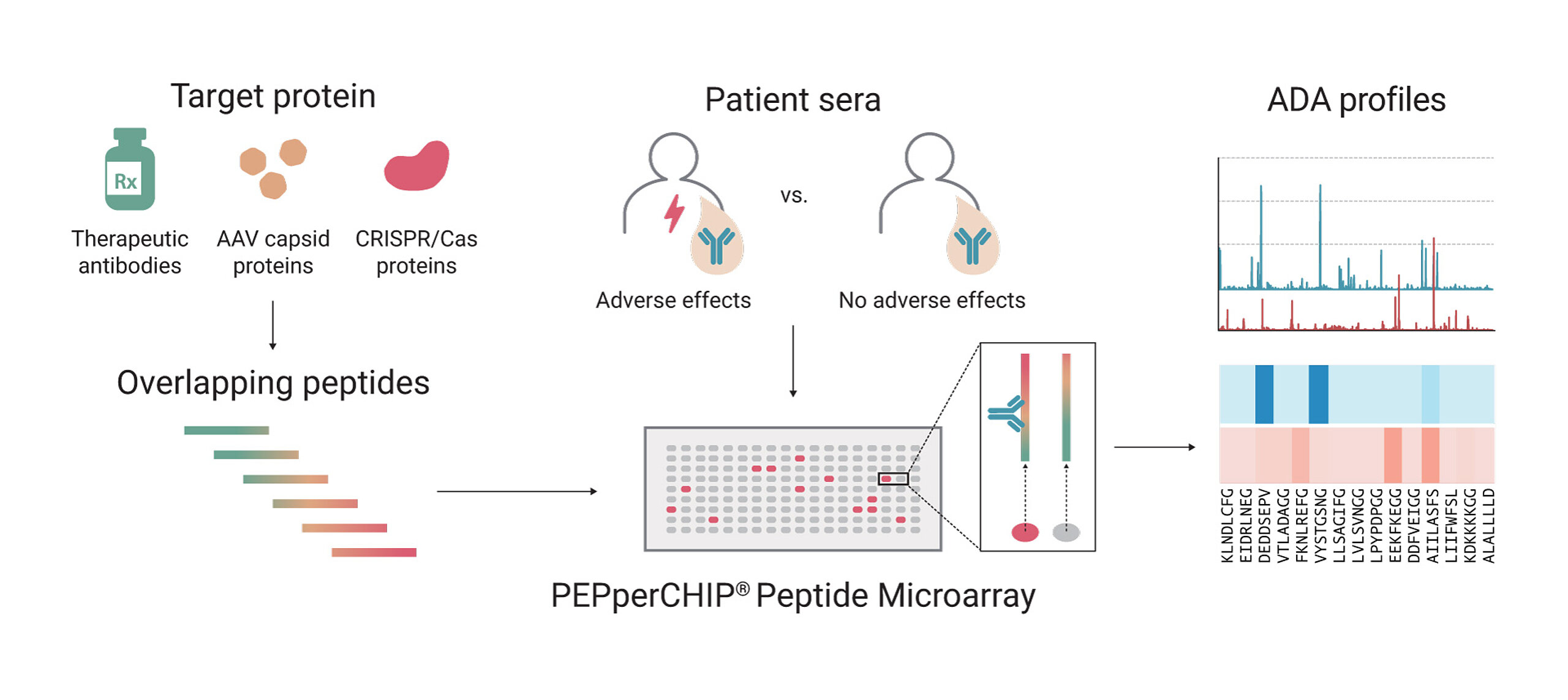
The overlapping peptide library approach ensures that every potential ADA-binding region is screened. Each peptide is typically 15 amino acids long with a +1 amino acid shift, enabling the evaluation of each amino acid’s effect on ADA binding. This high-resolution scanning allows researchers to pinpoint even minor immunogenic hotspots. Identification of these sequences can be leveraged for improving drug design or reducing unwanted ADA responses.
These responses are often mediated by T cells, which stimulate ADA production through co-signaling with APCs and B cells presenting antigenic peptides from the therapeutic protein. T cell-dependent ADA generation is a major concern in drug development, making epitope mapping an invaluable tool for identifying peptide sequences within the therapeutic protein that can either be optimized to reduce immunogenicity, or used as a prognostic marker for therapeutic outcomes.
Three case studies highlighting the importance of ADA epitope mapping
ADA epitope mapping is not only a critical step in the development of a drug, but also a versatile tool that can be useful in a number of different contexts during drug development. We have selected the following case studies to illustrate this versatility:
Case study 1: PD-L1 inhibitors atezolizumab vs. durvalumab
Over the past decade, PD-L1 inhibitors have become the standard of care for lung cancer patients, and atezolizumab and durvalumab are prime examples of this class of drugs, having been on the market since 2016 and 2017, respectively. According to a recent study, atezolizumab use is associated with a higher incidence of ADA development compared to durvalumab. To show this, we examined ADA responses in lung cancer patients treated with the two PD-L1 inhibitors.
The findings indicated that ADA responses were more pronounced against atezolizumab compared to durvalumab, suggesting a higher immunogenic potential for the former. Analysis of antibody isotypes revealed that IgG was the most dominant, whereas IgA and IgM responses were found to be minimal for the patient samples analyzed in this study.
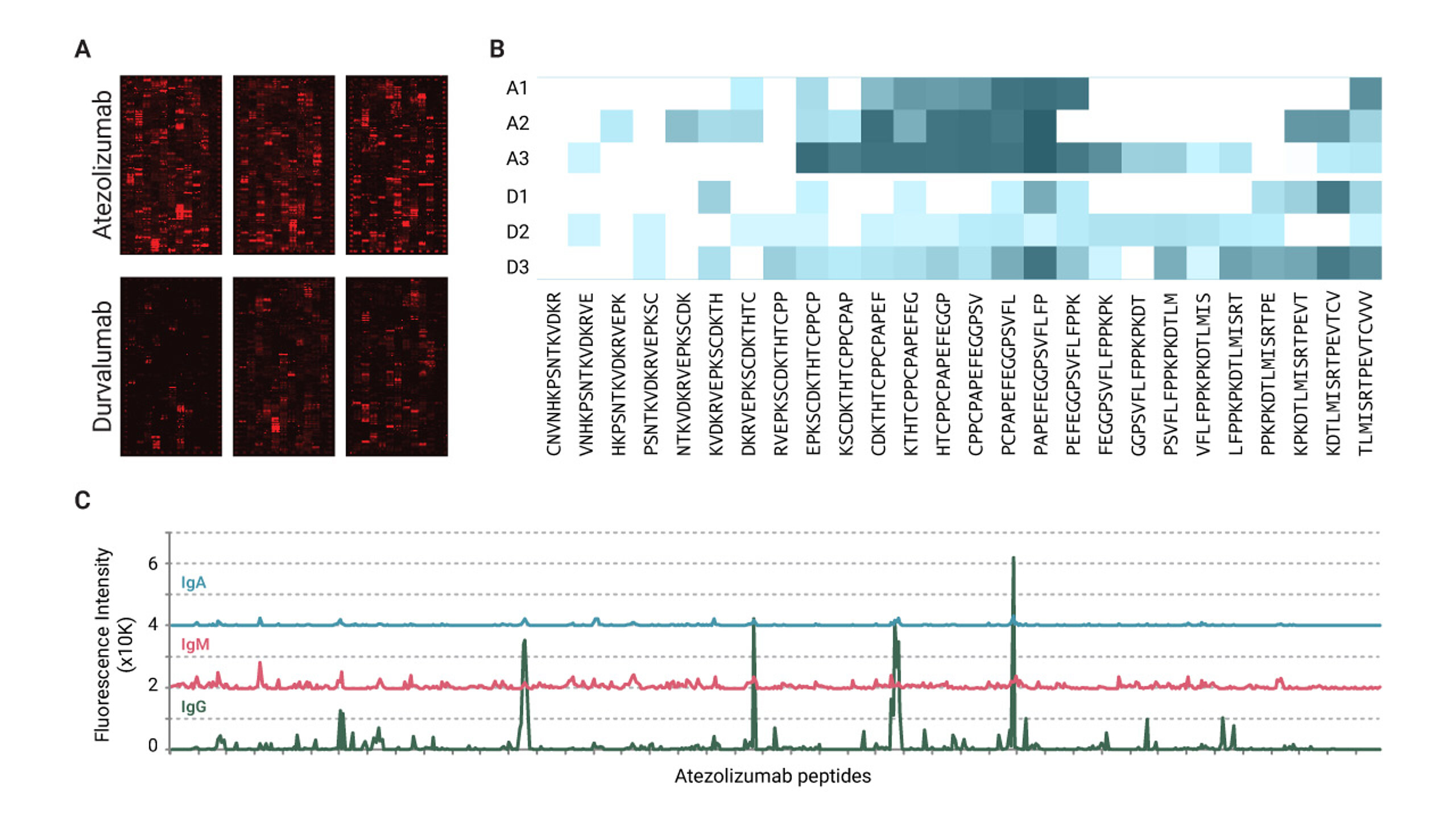
Expanding the study to a larger cohort could provide greater clarity regarding immunogenic differences between the two drugs. Certain epitope regions were identified as frequently recognized across multiple patients, which implies the presence of common immunogenic sites. These sites could potentially be modified to mitigate the risk of ADA development. These findings highlight the impact of structural variations in biologics on immunogenicity and illustrate how consideration of ADA epitope mapping early in drug development can provide a significant competitive advantage.
Case study 2: Brolucizumab and retinal vasculitis
Another case involved the anti-VEGF antibody brolucizumab, which was associated with rare but severe retinal vasculitis. Through ADA epitope mapping, researchers identified specific linear epitopes that were recognized by ADA in both treated and untreated patients, suggesting a prior exposure of the immune system to proteins with structural similarity.
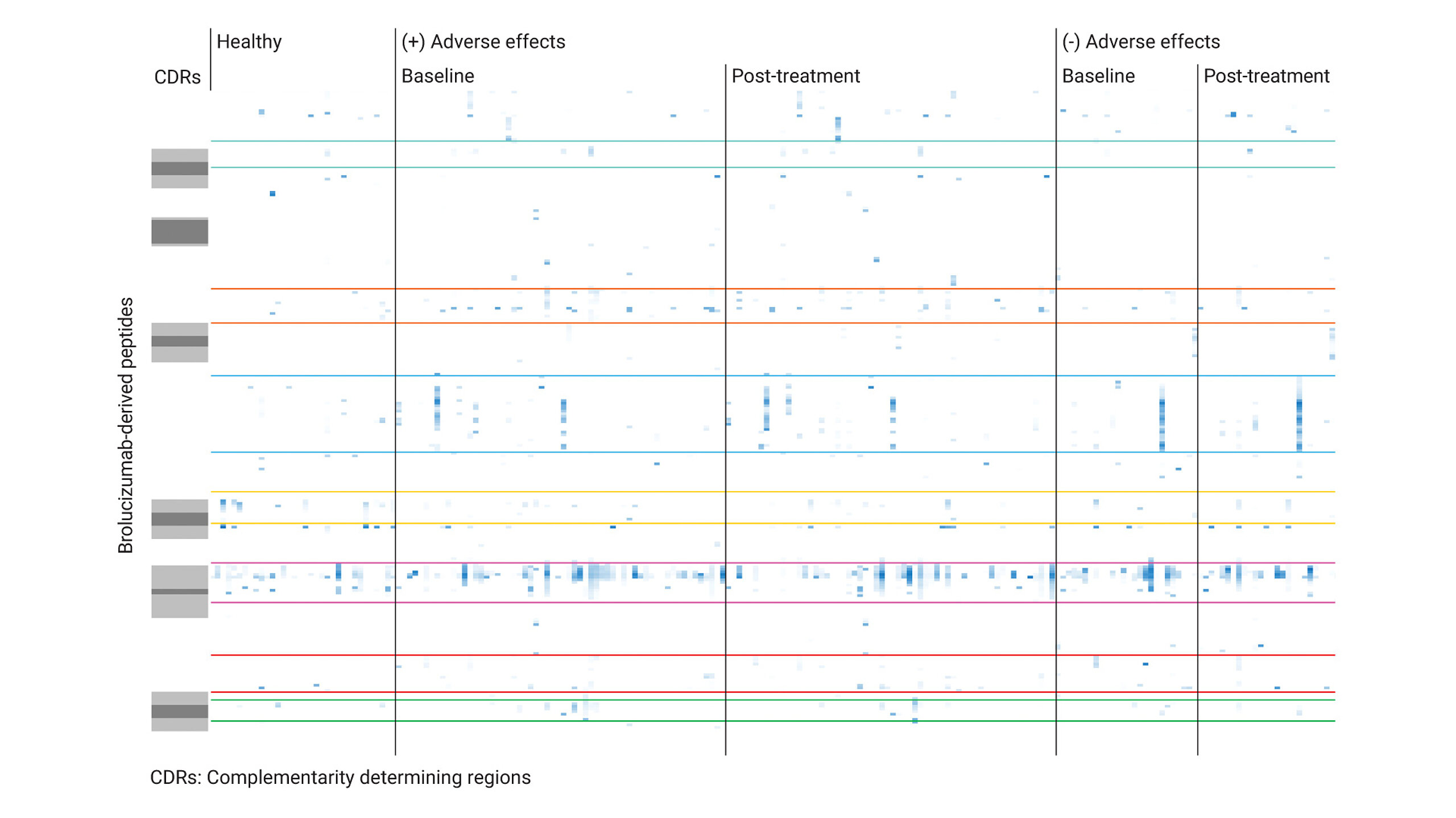
This analysis found that treated patients and untreated individuals with ADAs showed a polyclonal response against several linear brolucizumab epitopes. However, only patients who developed severe side effects showed a meaningful T-cell response upon re-exposure to the drug. Additionally, the study highlighted the potential to identify biomarkers that could help predict which patients are at greater risk for adverse reactions. This case reinforces the significance of ADA mapping as a predictive tool for identifying and mitigating rare but serious side effects associated with biotherapeutics.
Case study 3: Detecting traces of past bacterial encounters
CRISPR-based therapies hold great promise, but immune responses to bacterial Cas proteins could affect their safety and efficacy. Through ADA epitope mapping, our team screened sera from healthy individuals and discovered pre-existing IgG responses to selected CRISPR/Cas proteins.
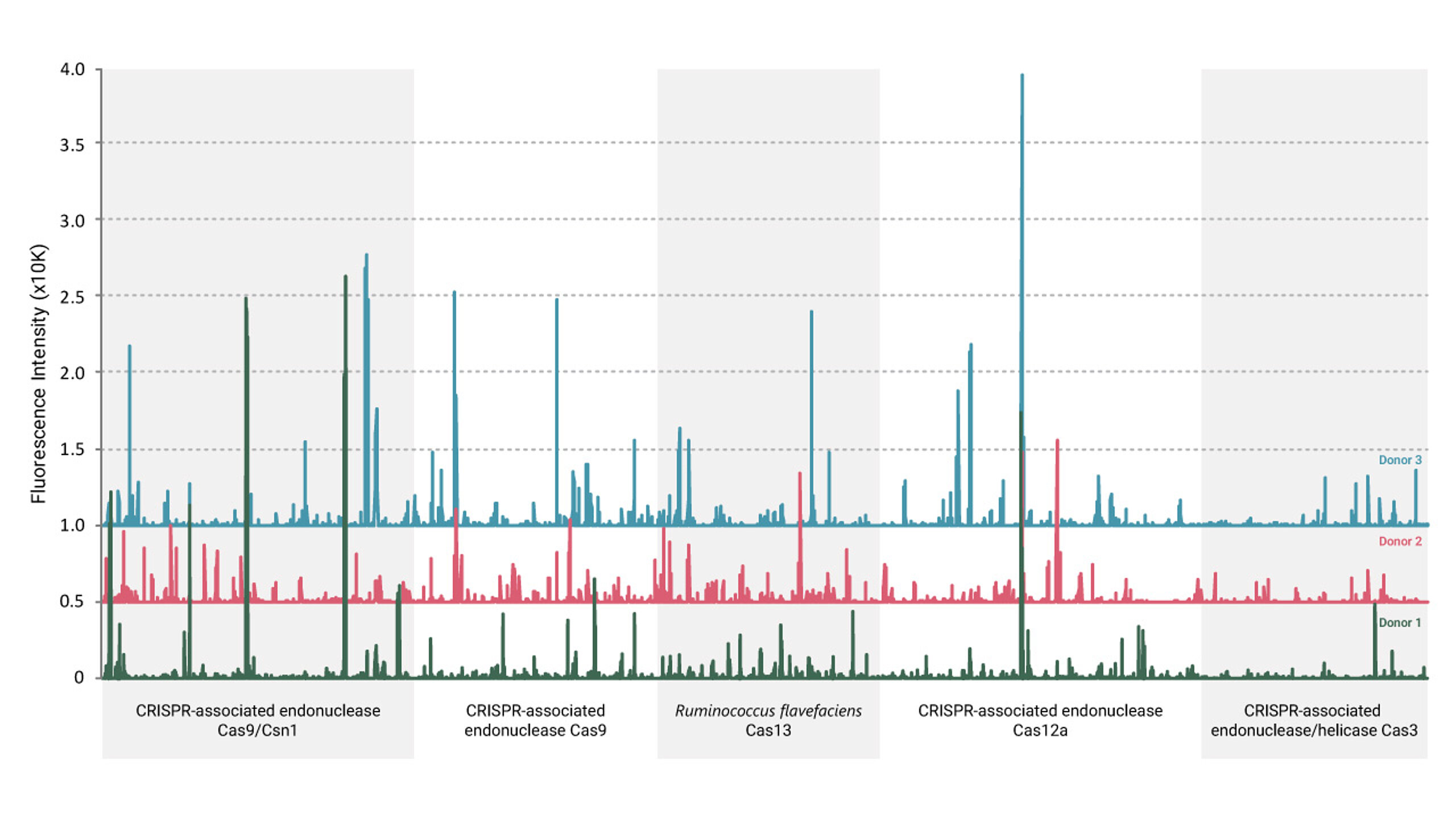
This finding suggests that some individuals may have been previously exposed to bacterial Cas proteins, leading to an immune memory that could interfere with CRISPR-based therapies. The study provided proof-of-concept data supporting the need for further investigation in larger patient cohorts. Understanding these pre-existing immune responses is critical for optimizing CRISPR-based therapeutic strategies and ensuring their long-term safety and effectiveness.
The future of ADA risk assessment in biopharmaceuticals
Anti-drug antibodies represent a significant challenge in the development of biologic therapeutics, but early-stage ADA risk assessment and epitope mapping provide powerful solutions to mitigate these risks. By identifying immunogenic epitopes, biopharmaceutical developers can refine drug formulations, minimize immune responses, and improve patient outcomes. As personalized medicine advances, ADA profiling will play an increasingly critical role in ensuring that innovative therapies reach their full potential while maintaining safety and efficacy.
As shown in the different case studies above, PEPperPRINT has been at the forefront of ADA epitope mapping thanks to our unique laser-printed microarray technology. If immunogenicity is a concern in your research, please contact us for a personal consultation.