Epitope Mapping
Understanding antibody epitopes
Epitope mapping is a crucial technique in immunology that determines the specific regions or sequences within an antigen that are reconized by an antibody. This information is invaluable for therapeutic, diagnostic, and research applications. Epitopes can be classified into two main types:
- Linear Epitopes consist of a continuous sequence of amino acids within the antigen. They are recognized by antibodies based on their primary structure, making them relatively straightforward to identfy.
- Conformational Epitopes are formed by amino acids that are not in a sequential order but are brought together by the folding of the protein. Recognition of conformational epitopes depends on the three-dimentional structure of the antigen, making their identification more complex. Conformational epitopes may also be discontinuous.
Our epitope mapping approach: How it works
PEPperCHIP® Peptide Microarrays are an excellent research tool that can be used to identify linear and conformational epitopes. Thanks to our platform technology, we can print overlapping linear or cyclic constrained peptides of any protein or antigen directly onto glass slides. Our microarrays can be generated with the maximum peptide overlap of shift +1, enabling highly resolved epitope data. Incubation of the resulting peptide microarray with an antibody or serum sample as well as suited secondary antibodies gives rise to spot patterns that correlate with the epitope of the given sample.

Benefits of epitope mapping with PEPperCHIP® Peptide Microarrays
- High Resolution: Allows identification of epitopes at the amino acid level.
- Full Flexibility: Customizable arrays enable the screening of thousands of peptides simultaneously.
- Cost-Effectiveness: Efficiently maps epitopes at a lower cost compared to other high-resolution methods.
LINEAR EPITOPE MAPPING
For identifying linear epitopes, we offer our standard epitope mapping format based on a single peptide length and maximum peptide overlap for high resolution epitope data. The approach is straightforward and is recommended for analyzing monoclonal antibodies and serum samples
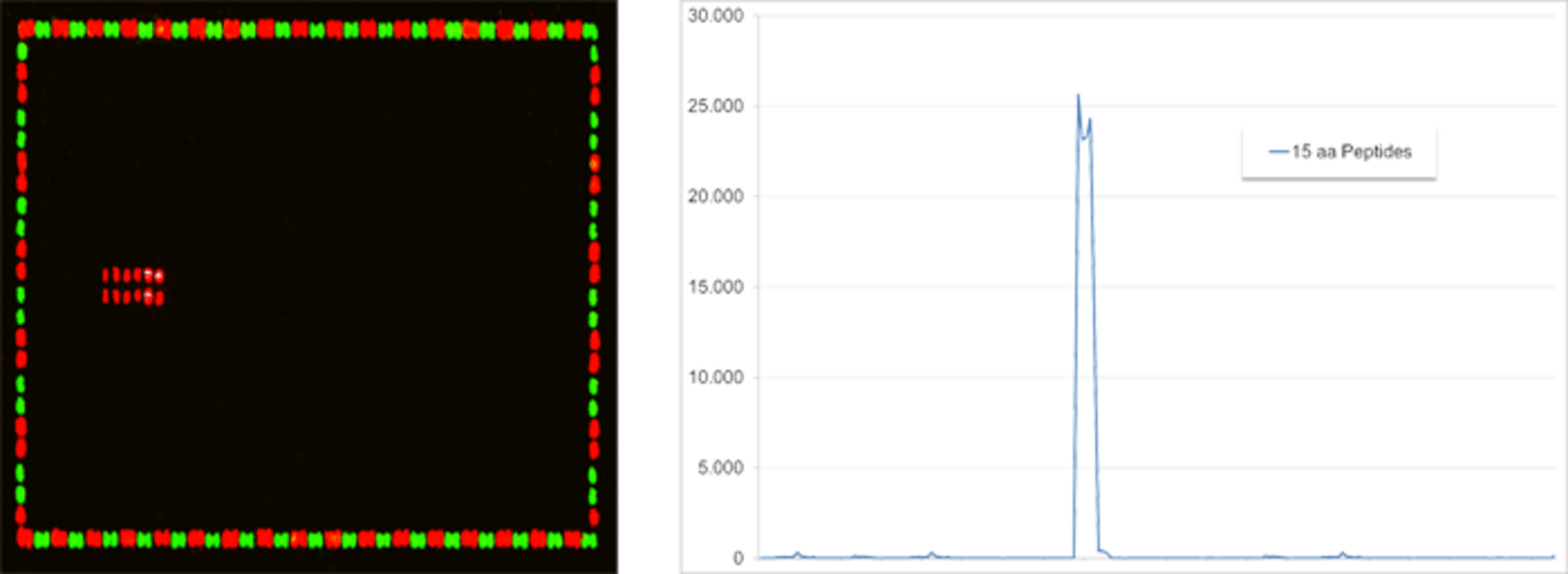
Linear Epitope Mapping of a monoclonal antibody against an antigen translated into 15 aa peptides with 14 aa peptide overlap. The microarray scan (left) as well as the intensity plot (right) reveal a stretch of adjacent peptides with a consensus motif that represents the epitope of the antibody. Stained HA (red) and Flag (green) control peptides frame the antigen-derived peptides.
Specifications
| Microarray content | Single to multiple antigens translated into overlapping peptides |
| Peptide length | 15 amino acids by default (adjustable) |
| Peptide overlap | 14 amino acids by default (adjustable) |
| Peptide replicates | Double spots by default (adjustable) |
| Control peptides | HA (YPYDVPDYAG) epitopes by default |
| Example | A protein sequence of 400 amino acids will be translated into 2 x 400 different peptides |
| Input data | Protein sequence(s) in FASTA format or with UniProt ID |
| Sample material | 10-20 µl serum or 10-20 µg purified antibody |
| Analysis | DIY following the PEPperCHIP® Immunoassay Protocol, or by PEPperMAP® Service |
CONFORMATIONAL EPITOPE MAPPING
Some antibodies bind to conformational epitopes, which are not readily mapped with linear peptides. PEPperCHIP® Peptide Microarrays can overcome this limitation with the generation of cyclic constrained peptides. With varying peptide lengths in an array, these cyclized peptides can mimick looped epitope structures, combining the advantages of PEPperPRINT's unrivalled peptide sequence flexibility with on-array generation of cyclic constrained peptides.
By using antigen-specific PEPperCHIP® Peptide Microarrays with cyclic constrained peptides, conformational epitope mapping is made possible and enables the characterization of monoclonal antibodies with conformational epitopes in a uniquely high epitope resolution.
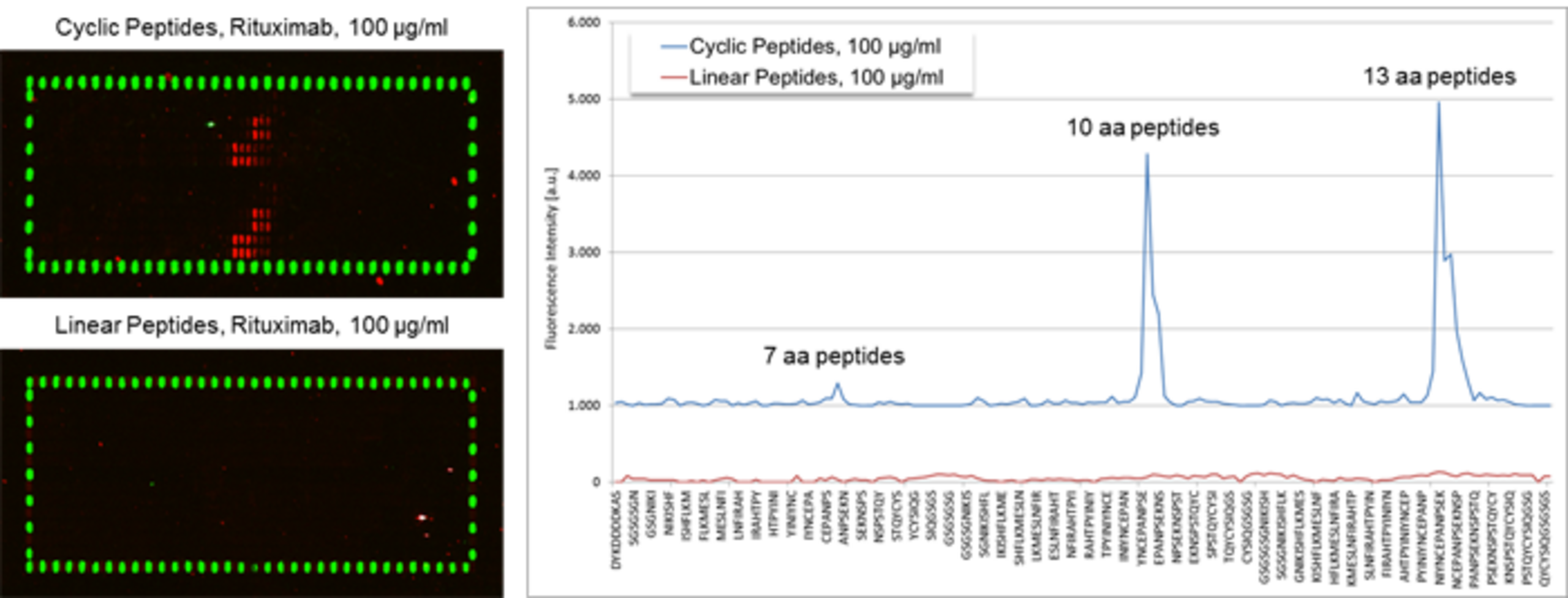
PEPperCHIP® Peptide Microarrays covering the extracellular domain of CD20 translated into overlapping linear and constrained cyclic peptides. The first double-row corresponds to the 7 amino acid peptides, the second and the third double-row to the 10 and 13 amino acid peptides. The arrays were assayed with 100 µg/ml of an anti-CD20 antibody followed by staining with anti-human IgG (red) and anti-Flag (green) antibodies. Linear peptides did not show any response against the antibody, whereas the constrained cyclic peptides exhibited clear epitope stretches. Flag control peptides framing the peptide array showed the expected well-defined spot pattern.
Specifications
| Microarray content | Single to multiple antigens translated into overlapping peptides |
| Peptide length | 7, 10, and 13 amino acids (adjustable) |
| Peptide overlap | 6, 9, and 12 amino acids by default (adjustable) |
| Peptide replicates | Double spots by default (adjustable) |
| Control peptides | HA (YPYDVPDYAG) epitopes by default |
| Example | A 400 amino acid protein is translated into 3 x 400 = 1200 peptides in duplicate |
| Input data | Protein sequence(s) in FASTA format or with UniProt ID |
| Sample material | Minimum 70 µl serum or 70 µg purified antibody |
| Analysis | DIY following the PEPperCHIP® Immunoassay Protocol, or by PEPperMAP® Service |
Epitope mapping use cases
Knowing epitope data is essential for several reasons. Some of these include: understanding antibody menchanisms of action, rational design of therapeutic or diagnostic products, and even the protection of intelectual property. Learn how epitope mapping with PEPperCHIP® Peptide Microarrays can accelerate your research by exploring some of our selected case studies and customer publications.
Application notes
- Validation of keratin antibodies by peptide mapping
- Characterization of an anti-CD20 antibody by conformational epitope mapping and epitope substitution scan
- Comparison of high resolution PEPperMAP® Epitope Mappings with low resolution epitope mappings
Poster
Cited publications
- ASFV epitope mapping by high density peptides microarrays (Desmet et al. 2024)
- SARS-CoV-2 Rapid Antigen Test Based on a New Anti-Nucleocapsid Protein Monoclonal Antibody: Development and Real-Time Validation (Coelho et al. 2023)
- Linear epitope mapping in the E and NS1 proteins of dengue and Zika viruses: Prospection of peptides for vaccines and diagnostics (Aquino et al. 2023)

